Compliance Recap | June 2020
June was a busy month in the employee benefits world.
The Internal Revenue Service (IRS) released the updated patient-centered outcomes research institute (PCORI) fee amount and announced transition relief. The Department of Health and Human Services’ Office for Civil Rights (OCR) published a final rule regarding the Patient Protection and Affordable Care Act’s (ACA’s) Section 1557.
The President signed the Paycheck Protection Program Flexibility Act of 2020. The Department of Labor (DOL), Department of Health and Human Services (HHS), and the Department of the Treasury (Treasury) (collectively, the Departments), issued additional frequently asked questions (FAQs) on health plan coverage under the Families First Coronavirus Response Act (FFCRA) and the Coronavirus Aid, Relief, and Economic Security Act (CARES Act).
The Centers for Medicare and Medicaid Services (CMS) issued a letter highlighting COVID-19 guidance for non-federal governmental plan sponsors. CMS issued an information bulletin for insurers regarding the 2019 medical loss ratio (MLR) rebate.
The IRS released a proposed rule on direct primary care, health care sharing ministries, and other medical arrangements. The DOL released proposed updates for the 2020 Mental Health Parity and Addiction Equity Act of 2008 (MHPAEA) Self-Compliance Tool. The Congressional Research Service (CRS) issued an updated version of its Health Insurance Continuation Coverage Under COBRA report. CRS also provided an overview of potential COVID-19 impacts on health flexible spending accounts (FSAs) and recent health FSA changes.
The IRS issued Notice 2020-35, which provides additional relief in the form of postponed deadlines for certain time-sensitive actions due to the COVID-19 emergency. The DOL issued a field assistance bulletin on an employee’s eligibility for leave under the FFCRA due to the closure of summer programs for an employee’s child.
IRS Releases Updated PCORI Fee and Transition Relief
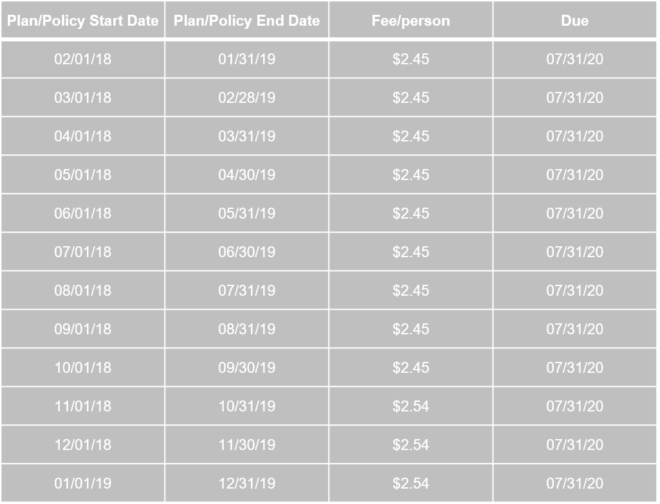
The IRS released Notice 2020-44 (Notice) that provides the PCORI fee amount is $2.54 per covered life for plan years that end on or after October 1, 2019, and before October 1, 2020. The Notice also provides that plan sponsors may continue to use the actual count method, the snapshot count method, or the Form 5500 method for calculating the average number of covered lives. Additionally, the Notice provides that a plan sponsor may use any reasonable method for calculating the average number of covered lives for plan years ending on or after October 1, 2019, and before October 1, 2020. If a plan sponsor uses a reasonable method to calculate the average number of covered lives for plan years ending on or after October 1, 2019, and before October 1, 2020, then that reasonable method must be applied consistently for the duration of the plan year. The fee is due by July 31 of the year following the calendar year in which the plan/policy year ends.
Read more about the PCORI fee.
OCR Publishes Final Rule to Revise ACA Section 1557 Regulations
The Department of Health and Human Services’ Office for Civil Rights (OCR) published a final rule, fact sheet, and press release that revise its regulations under the Patient Protection and Affordable Care Act’s Section 1557. The final rule takes effect August 18, 2020.
The final rule eliminates:
- Certain definitions, including the definition of “covered entity”
- Specific nondiscrimination definitions based on sex and gender identity
- Translated taglines in significant consumer communications, the requirement to post information about Section 1557 and nondiscrimination at a covered entity’s locations and website, use of language access plans, and certain video standards for individuals with limited English proficiency (LEP)
- Any reference to a private right of action to sue covered entities for violations of the proposed rule
- The requirement to have a compliance coordinator and written grievance procedure to handle complaints about Section 1557 violations
- Enforcement-related provisions
Due to state nondiscrimination requirements and the U.S. Supreme Court’s decision in Bostock v. Clayton County Board of Commissioners on June 15, 2020, ruling that termination of an employee for being gay or transgender violates Title VII of the Civil Right Act, employers should consult with their counsel before removing protections under the health plan in reliance on the final rule.
Read more about the final rule.
President Signs Paycheck Protection Program Flexibility Act of 2020
On June 5, 2020, President Trump signed the Paycheck Protection Program Flexibility Act of 2020 (PPPFA), which amended the Paycheck Protection Program established under the Coronavirus Aid, Relief, and Economic Security Act (CARES Act). The PPPFA extended the maturity date of PPP loans to at least five years for loans made on or after June 5, 2020. The PPPFA extended the application period for a PPP loan from June 30, 2020, to December 31, 2020. However, the Small Business Administration announced that it will not accept applications after June 30, 2020. Under the PPPFA, the loan forgiveness period was extended from eight weeks to 24 weeks, but no later than December 31, 2020. However, if an employer received a covered loan before June 5, 2020, the employer may elect for the covered period to end eight weeks after the origination date of the loan. The PPPFA extends the date, from June 30, 2020, to December 31, 2020, by which employers must eliminate a reduction in employment or salary and wages that would otherwise reduce the amount of loan forgiveness the employer is entitled to. The PPPFA also extends the principal and interest payment deferral period for PPP loans.
Read more about the PPP.
DOL, HHS, and the Treasury Issue additional FAQs on Health Plan Coverage under the FFCRA and the CARES Act
The DOL, HHS, and the Treasury (collectively, the Departments), issued FAQs regarding implementation of the Families First Coronavirus Response Act (the FFCRA), the Coronavirus Aid, Relief, and Economic Security Act (the CARES Act), and other health coverage issues related to Coronavirus Disease 2019 (COVID-19). These FAQs are in addition to the FAQs the Departments previously released regarding health plan coverage under the FFCRA and CARES Act.
Read more about the FAQs.
CMS Issues Letter Highlighting COVID-19 Guidance for Non-Federal Governmental Plan Sponsors
CMS issued a letter highlighting COVID-19 guidance relevant to non-federal governmental plan sponsors. The letter highlights guidance that has previously been released and does not issue any new requirements. The letter highlights 1) the requirement to cover COVID-19 diagnostic testing and certain related items and services without cost-sharing or medical management, 2) the temporary period of relaxed enforcement of certain timeframes under the Public Health Service Act, 3) the expansion and promotion of access to telehealth options and prescription drugs during the COVID-19 outbreak, and 4) enforcement relief from the 60-days’ advance notice requirement for certain mid-year plan changes.
Read more about the letter.
CMS Issues Information Bulletin for Insurers Regarding the 2019 MLR Rebate
The Centers for Medicare and Medicaid Services (CMS) issued an information bulletin for insurers regarding the 2019 MLR rebate. The bulletin provides insurers with flexibilities for providing policyholders the 2019 Medical Loss Ratio (MLR) rebates. CMS will not take enforcement action against insurers that elect to pay a portion or all of its estimated 2019 MLR rebate in the form of a premium credit prior to September 30, 2020, and in advance of filing its 2019 MLR Annual Reporting Form (August 17, 2020). An insurer that chooses to provide the rebate via a lump-sum check or lump-sum reimbursement to the account used to pay the premium may also pay a portion or all of its estimated 2019 MLR rebate in advance of filing the 2019 MLR Annual Reporting Form, under the conditions outlined in the bulletin. After filing the 2019 MLR Annual Reporting Form, if an insurer that prepaid a portion or all of its estimated 2019 MLR rebate determines that such prepayment exceeds the total 2019 rebate amount owed, the insurer can include the overage when calculating the limit on the rebate amount payable for 2020 or 2021, if applicable. This may enable an insurer to reduce the 2020 or 2021 rebate payment by approximately the amount of the 2019 rebate overpayment.
IRS Releases Proposed Rule on Direct Primary Care, Health Care Sharing Ministries, and Other Medical Arrangements
The Internal Revenue Service (IRS) under the Department of the Treasury (Treasury) released a proposed rule on treating payments for direct primary care (DPC) arrangements and health care sharing ministry arrangements as Internal Revenue Code Section 213(d) medical expenses for purposes of health reimbursement arrangements (HRAs) and health savings accounts (HSAs), and when such arrangements would be disqualifying coverage for HSAs. The proposed rule would also clarify that payments for other arrangements qualify as Section 213(d) medical expenses, including health maintenance organizations (HMOs), Medicare Parts A, B, C, and D, Medicaid, Children’s Health Insurance Program (CHIP), TRICARE, and certain veterans’ health care programs. Public comments on the proposed rule are due August 10, 2020.
DOL Releases Proposed Updates for the 2020 MHPAEA Self-Compliance Tool
The DOL released proposed updates to its 2018 MHPAEA Self-Compliance Tool and issued a press release. The DOL publishes the MHPAEA Self-Compliance Tool to help group health plan sponsors and administrators, as well as other entities, determine whether a group health plan or health insurance issuer is in compliance with the MHPAEA and its regulations. The Mental Health Parity and Addiction Equity Act of 2008 (MHPAEA) generally requires that the financial requirements and treatment limitations imposed by a group health plan or health insurance issuer on mental health and substance use disorder benefits cannot be more restrictive than the predominant financial requirements and treatment limitations that apply to substantially all medical and surgical benefits.
The proposed updates would integrate prior guidance published in FAQs on the implementation of the MHPAEA. The proposed updates would add explanations of how plans and issuers could correct compliance violations and would provide additional examples of how to comply with MHPAEA. The proposed updates would provide best practices for establishing an internal compliance plan and would provide examples of the types of records that a plan or issuer may need to provide in the event of a DOL investigation. Finally, the proposed updates would provide additional examples of treatment limitations encountered in recent federal and state investigations that may be warning signs of a potential violation. Public comments on the proposed updates are due July 24, 2020.
Read more about the FAQs.
CRS Updates Overview of Health Insurance Continuation Coverage Under COBRA
The CRS issued an updated version of its Health Insurance Continuation Coverage Under COBRA report. This report provides a general background on COBRA, explains who qualifies for COBRA, and provides an overview of employer and employee responsibilities under COBRA. This updated report includes descriptions of the temporary relief provided under the final rule issued by the DOL and IRS extending certain COBRA timeframes in response to the COVID-19 pandemic. The updated report also includes an overview of bills that have been introduced in Congress that would provide premium assistance to qualified beneficiaries (and to furloughed employees who have not lost coverage under some of the bills) in response to the COVID-19 pandemic. The updated report also includes information on the interaction of COBRA and Medicare.
See our Advisors “Final Rule on the Extension of Certain Timeframes for Employee Benefit Plans, Participants, and Beneficiaries Due to COVID-19” and “Updated Department of Labor Model COBRA Notices.”
CRS Provides Overview of Potential COVID-19 Impacts on Health FSAs and Recent Health FSA Changes
The CRS issued a report on the potential COVID-19 impacts on health flexible spending arrangements (health FSAs) and recent health FSA changes. The report provides a brief overview of health FSAs and some potential ways the COVID-19 pandemic may affect health FSAs. The report also reiterates recent guidance issued by the IRS – Notice 2020-29 and Notice 2020-33 – that provide employers with the opportunity to offer two types of flexibilities to employees enrolled in the employer’s health FSA. Employers may provide employees with the ability to make prospective, midyear changes (during calendar year 2020) to the amount the employee contributes to his or her health FSA (includes enrolling in a health FSA or revoking a health FSA election). In addition, employers that offer health FSAs with plan years (or grace periods) that end in 2020 may extend the time frame in which employees may access such funds through December 31, 2020. Under the CARES Act, qualified medical expenses for health FSA reimbursement was expanded to include over-the-counter medicines and drugs without a prescription and menstrual care products.
See our Advisors “Internal Revenue Service Notice 2020-29”, “Internal Revenue Service Notice 2020-33”, and “The Coronavirus Aid, Relief, and Economic Security Act FFCRA Amendments and Benefits Provisions”.
DOL Issues Field Assistance Bulletin on FFCRA Leave due to Closure of Summer Programs
The DOL issued a field assistance bulletin (FAB) providing guidance on when an employee may take leave under the FFCRA to care for his or her child based on the closure of a summer camp, summer enrichment program, or other summer program for COVID-19 related reasons. Under the FFCRA, leave may be taken if an employee is unable to work or telework due to the need to care for his or her child whose place of care is closed due to COVID-19 related reasons. The DOL recognizes summer camps and programs may qualify as “places of care” for purposes of FFCRA leave even if they would not have been operating at the time FFCRA regulations were issued in April 2020. DOL investigators will consider whether there is evidence of a plan for the child to attend the camp or program, or whether it is more likely than not that the child would have attended the camp or program had it not closed due to COVID‑19. Affirmative steps short of enrollment in the camp or program may be sufficient. Examples of affirmative steps may include submission of an application or deposit before the camp or program closed. Also, a child’s prior attendance and current eligibility for a camp or program may be sufficient. Because of the multitude of possible circumstance in which an employee can establish a summer camp or program as a child’s place of care, the DOL does not provide a bright-line rule. However, a parent’s mere interest in a camp or program will generally not be enough.
Question of the Month
- Who must pay the Patient-Centered Outcomes Research Institute (PCORI) fee and when is the fee due?
- The fee must be determined and paid by:
- The insurer for fully insured plans (although the fee likely will be passed on to the plan)
- The plan sponsor of self-funded plans, including HRAs
- The plan’s TPA may assist with the calculation, but the plan sponsor must file IRS Form 720 and pay the applicable fee
- If multiple employers participate in the plan, each must file separately unless the plan document designates one as the plan sponsor
The fee is due by July 31 of the year following the calendar year in which the plan/policy year ends. For example:
7/1/2020